It is well known that the Food Safety and Inspection Service’s (FSIS) primary responsibility is to ensure the safety of our nation’s meat and poultry supply. This does not mean, however, it takes its other responsibilities less seriously, including its responsibility to ensure meat and poultry products are properly labeled and labels do not bear false or misleading information. This became abundantly clear in recent meetings with industry leaders, in which agency officials announced several new labeling initiatives.
First, the agency announced it will soon audit generically approved labels at inspected establishments to verify that they are properly classified as generically approved. Pursuant to FSIS regulations, only labels that bear claims and statements defined by agency regulations and the Food Standards and Labeling Policy Book (excluding natural and negative claims) are deemed generically approved. Labels that bear “special statements or claims” — defined as natural and negative claims and claims that are not defined in FSIS regulations or Food Standards and Labeling Policy Book (e.g., animal production claims, health claims, ingredient and processing method claims, organic claims) — must be submitted to FSIS for evaluation and formal approval. FSIS is concerned that because of marketing efforts to make their products more appealing, establishments are rushing to make new claims and statements without submitting them for the required agency approval.
FSIS is conducting a pilot at a few inspected establishments to determine whether they properly classify labels as generically approved. Depending on the results, FSIS will determine whether to expand the audit nationally. In any event, officials said the agency will prepare new training materials for its inspection personnel regarding generic approval and will work on more routine verification activities to ensure labels bearing special claims and statements are properly approved.
Second, the agency intends to expand laboratory verification programs to ensure establishments are making truthful statements and claims on labels. For example, FSIS currently samples raw ground beef products for nutrient analysis in order to verify nutrition labeling requirements. In light of the fact FSIS has found some instances of gross variations with respect to fat and sodium declared on labels of ground beef products, FSIS intends to expand its nutrient sampling program to include poultry and other products. FSIS will also take a closer look at additional label claims that can be verified by laboratory-based methods. FSIS intends to finalize plans to collect samples of products that bear claims such as “never fed antibiotics” and test them to ensure the claims are truthful. Similar testing plans will be developed for products that claim “never fed hormones” and or that the products are free of allergens.
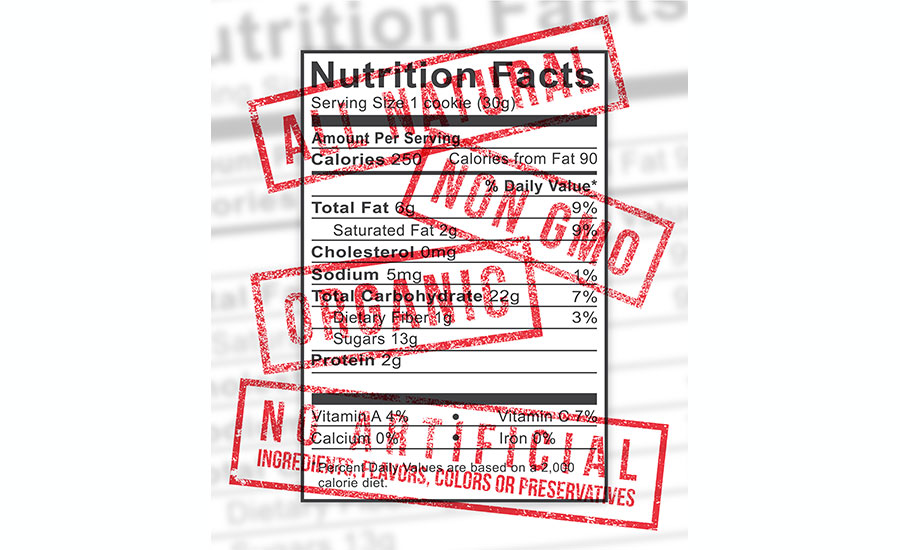
Third, FSIS announced it will issue a proposed new definition for “natural” this year. Pursuant to the current FSIS Standards and Labeling Policy Book, products can be labeled “natural” if the product and ingredients are: (1) minimally processed and (2) do not contain any artificial or synthetic ingredient, coloring ingredient or chemical preservative. It is not clear whether either of these requirements will be maintained.
Finally, the agency expects to soon issue a proposed rule to modify the nutrition facts panel for meat and poultry products and to update certain reference amounts customarily consumed. The proposed rule went to OMB on July 25 and is expected to closely track the final nutrition facts rule that FDA issued in May.
Food safety should continue to be the primary concern for all meat and poultry establishments. But with FSIS poised to implement the above initiatives in the near future, establishments would be well advised to spend some time ensuring their labels comply with agency requirements designed to ensure truthful and accurate labeling. NP




Report Abusive Comment