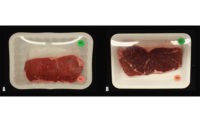
A greater concentration of oxymyoglobin in meat results in consumer-preferred, bright, cherry-red color of beef. However, exposing meat to air results in conversion of oxymyoglobin into metmyoglobin, and consequently in the formation of very dark-red discoloration of the meat. Several factors, including oxygen partial pressure, influence oxymyoglobin oxidation stability.
High-oxygen modified packaging (HiOx-MAP; 80% oxygen content) is utilized by the meat industry to maximize oxygen partial pressure in beef steaks and increase their shelf-life. As a result, the average shelf-life of steaks in HiOx-MAP is 10-12 days compared to 5-7 days in PVC/atmospheric oxygen partial pressure (20% oxygen) at 4°C. Steaks aged for 7-14 days and packaged in HiOx-MAP show less discoloration, but more lipid oxidation than PVC packaged steaks aged for the same period. This raises a question regarding the preservative effects of HiOx-MAP, because high-oxygen content increases lipid oxidation products, and these compounds are known to stimulate oxymyoglobin oxidation.
An in-vitro study using equine myoglobin (it has about 88.9% structural similarity with bovine myoglobin) was conducted to evaluate the combined effects of 4-hydroxyl-2-nonenal (HNE) and oxygen partial pressure on myoglobin oxidation.
Oxymyoglobin samples treated with / without HNE, were incubated under controlled high-oxygen partial pressure (80% O2) or atmospheric partial pressure (20% O2). The simulated retail display utilized was a coffin-style display case maintained at 2±1 °C under continuous fluorescent lighting for 0, 48, and 96 hours. At the end of each incubation period, metmyoglobin formation, dissolved oxygen saturation and HNE binding with myoglobin were determined under the two oxygen partial pressure conditions. By the end of the incubation period at 96 hours, HNE treatments in 80% oxygen content resulted into greater oxymyoglobin oxidation compared to 20% oxygen content treatments. Additionally, high-oxygen packaged oxymyoglobin solutions had greater dissolved oxygen saturation (15.5 vs.7.5 ppm) compared to atmospheric oxygen packaged oxymyoglobin solutions (standard error = 0.5; P < 0.05). Thus, high-oxygen packaging created greater oxygen levels in oxymyoglobin solutions incubated under high oxygen content compared to atmospheric oxygen conditions.
The solubility of oxygen is greater at lower temperatures favoring free radical formation in the presence of a catalyst such as light. For example, in the present study, myoglobin samples were kept under continuous fluorescent lighting to simulate retail display conditions. Therefore, ultraviolet rays in display light, in part, can act as a pro-oxidant and initiate oxymyoglobin oxidation. Additionally, the current in-vitro system did not involve metmyoglobin reducing activity; hence a balance between antioxidant and pro-oxidant processes in-situ may determine meat discoloration. However, oxygen levels did not significantly impact rates of HNE binding to myoglobin under the conditions tested. Hence, we speculate that the combined effects of high-oxygen conditions and HNE-binding may have accelerated oxymyoglobin oxidation with increased incubation time.
In summary, high-oxygen conditions can improve the formation of desired bright-red color of the meat but at the same time those conditions can also increase lipid oxidation, that in turn, promotes formation of metmyoglobin and meat discoloration. Hence, packaging aged steaks in high-oxygen condition can be detrimental to meat color. NP



Report Abusive Comment