At the point of sale, consumers often use the cherry-red color of beef as an indicator of wholesomeness and quality of the product. Meat discoloration, which mainly results from oxidative browning, leads to consumer rejection and huge economic loss for the beef industry. Fresh meat color stability is determined by the postmortem interactions between myoglobin and several other biomolecules in skeletal muscle.
Post-translational modifications (PTM) are the covalent, and generally enzymatic, changes in proteins following protein biosynthesis by the addition or the removal of modifying group(s) at the amino acid level. PTM play a fundamental role in modulating protein’s functionality, turnover, and interactions with other proteins.
The complex relationship between PTM of muscle proteins and meat quality has become increasingly evident. However, myoglobin PTM and their role in fresh meat color stability has not been characterized. We hypothesized that PTM in myoglobin can influence the protein’s structural and functional properties and its interaction with other biomolecules, and in turn influence fresh meat color stability. Therefore, our objectives were to identify myoglobin PTM sites in beef longissimus lumborum muscle during postmortem aging and their influence on fresh beef color stability.
The longissimus lumborum muscle was collected from nine (n=9) beef carcasses obtained from the USDA-inspected meat laboratory at the University of Kentucky (Lexington, Ky.), and were divided into four equal-length sections. The muscle sections were vacuum packaged and randomly assigned to wet-aging at 2°C for either 0, 7, 14, or 21 days. At the end of each wet-aging period, the muscle sections were removed from their vacuum packages and fabricated into four 1.92-cm thick steaks. One steak from each muscle section allotted for proteome analyses was then immediately vacuum packaged and frozen at –80°C until it was used. The remaining three steaks assigned for evaluation of instrumental color and biochemical evaluation were aerobically packaged and assigned to refrigerated storage (2°C) in the dark for either 0, 3, or 6 days. Myoglobin PTM were analyzed using two-dimensional electrophoresis. Gels were stained for phosphorylated protein and total protein using Pro-Q Diamond and Sypro Ruby, respectively. The protein spots with molecular weight of 17 kDa were excised and subjected to tandem mass spectrometry for PTM identification. The instrumental color and biochemical properties were analyzed as a split-plot design with aging time as a whole-plot factor, and storage day as a sub-plot factor. The carcass was considered as a random effect. The data were analyzed using PROC MIXED procedure in SAS, and the differences among the means were detected using the least significant differences at a P < 0.05 level.
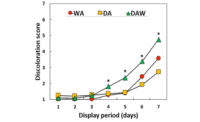
In agreement with previous investigations, fresh beef color traits such as redness (a* value), color stability (R630/580), and myoglobin concentration decreased (P < 0.05) upon aging. Gel image analysis identified six myoglobin spots with similar molecular weight (17kD) but different isoelectric pH. Our observation indicated that the six myoglobin isoforms were post-translationally modified to different degrees, since PTM can change proteins’ isoelectric pH by modifying titratable groups in amino acids. Tandem mass spectrometry identified multiple PTM (phosphorylation, methylation, carboxymethylation, acetylation, and hydroxynonenal alkylation) in the six myoglobin spots. Phosphorylation was detected in serine (S), threonine (T), and tyrosine (Y) residues, whereas lysine (K), arginine (R), and histidine (H) were susceptible to other PTM. In addition, distal histidine (histidine 64) which is critical to heme stability was modified by hydroxynonenal alkylation. Phosphorylation, acetylation, and carboxymethylation introduce the negatively charged phosphate group, acetyl group, and carboxylic acid, respectively, in myoglobin, which could alter the ionic network, and therefore might influence the distal histidine’s spatial interaction with hydrophobic heme pocket. While methylation and hydroxynonenal alkylation contribute to minimal change in isoelectric pH of myoglobin, they increase the hydrophobicity and stearic hindrance, and thus alter myoglobin’s tertiary structure.
Overall, greater number of myoglobin PTM sites were identified in aged beef (wet-aging for 7, 14, or 21 days) compared with non-aged counterparts (wet-aging for 0 days). These aging-induced PTM, especially those located close to the hydrophobic heme pocket, could compromise myoglobin redox stability by adding modifying groups to amino acid residues, and therefore accelerate myoglobin oxidation and beef discoloration. Interestingly, differential alkylation sites between non-aged and aged beef were identified at lysine residues, indicating a potential role of lysine alkylation in myoglobin redox stability. Furthermore, PTM at K45, K47, and K87 were unique to myoglobin from non-aged beef, whereas PTM at R31, T51, K96, K98, S121, R139, and K147 were unique to myoglobin from aged counterparts (Figure 1). These myoglobin PTM could be used as novel biomarkers for fresh beef color stability. The knowledge of protein PTM may be combined with artificial intelligence and machine learning to predict the color stability of beef muscles, and this approach could be utilized in production lines to optimize the value and to minimize food waste. Moreover, innovative processing strategies to improve meat color stability could be developed by minimizing myoglobin PTM-induced meat discoloration. For instance, future research would be valuable in understanding the influence of enzymes on meat color stability through reversing myoglobin PTM. Further research is required to determine the role of myoglobin PTM in internal cooked color to improve the safety of cooked ground beef.
For more information, please see the authors' work published in Meat and Muscle Biology: Yifei Wang, Shuting Li, Gregg Rentfrow, Jing Chen, Haining Zhu, Surendranath P. Suman, “Myoglobin Post-Translational Modifications Influence Color Stability of Beef Longissimus Lumborum” Meat and Muscle Biology 5(1). p.15, 1-21. Doi: https://doi.org/10.22175/mmb.11689


Report Abusive Comment