Wherever there are bacteria, very likely there are bacteriophages attacking them. So it makes sense to enlist nature’s predator to fight foodborne pathogens. Why are bacteriophages, then, still slow to the market?
Well, because other pre-harvest interventions like lactic acid can do the same job essentially — ensure the cleanliness of the animals and raw materials entering processing facilities — at a much less expensive price. The last 10 to 12 years have certainly seen an increase in companies researching and launching commercial applications of bacteriophages, or phages, but they are still held back by efficacy, cost and lack of market demand.
“The use of phages as antimicrobials is still in its infancy,” says Lawrence Goodridge, Ph.D., assistant professor of food microbiology, Colorado State University, Fort Collins, Colo. “It has been approximately eight years since phages were first approved for use on food to reduce the presence of spoilage and pathogenic bacteria. Since then a number of additional products have been approved for use as biopesticides and as antimicrobials to reduce the presence of pathogenic bacteria on food, and on food-producing animals.”
The National Chicken Council, for one, is supportive about the possibility of using bacteriophages in the chicken industry and encourages ongoing research and development of this technology.
“However, it is important that we emphasize that using bacteriophages is only one step in a multi-hurdle approach to improve food safety for our customers,” says Ashley Peterson, Ph.D., National Chicken Council, vice president of scientific and regulatory affairs, based in Washington, D.C. “There is no single solution to pre-harvest controls, but we are always looking to improve food safety through science-based and statistically validated approaches.”
How they work
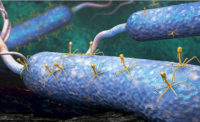
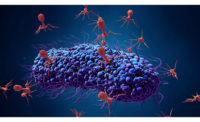
Phages are viruses that specifically infect bacteria.
“Like any virus, they take over their host cell [in this case, bacteria] and turn it into a virus-making factory,” says Goodridge. “One phage infects one bacterial cell, and as many as 5,000 phages can be made within the cell.”
Phages use the cellular mechanism of bacteria to make new phages, says John Glisson, Ph.D., director for research of the US Poultry and Egg Association’s Harold E. Ford Foundation, Tucker, Ga. “In this reproductive process, the bacteria is lysed and dies.”
After the phages are made, the cell bursts open releasing all the newly formed phages, allowing these phages to infect other bacterial cells — repeating the process.
For more than 80 years, phages have been used for medical purposes in Russia, Georgia and Poland, amongst other countries, notes Jason Gill, Ph.D., assistant professor of bacteriophage biology and microbiology, Texas A&M University, based in College Station, Texas.
“They were used in the West from the 1920s to the 1940s, but discontinued here after the mid-1940s,” he says. “Western interest in phages has been renewed over the last 10 to 15 years or so.”
Phages are getting a second look from processors as they look for alternatives to antibiotics, which have broad host ranges and will not only kill the target bacteria but good bacteria, as well.
“Phages are often specific only for the target bacteria in question, meaning that good bacteria will not be killed,” says Goodridge. “Phages are natural, and they are found everywhere in the environment that bacteria are found [including on and in humans, such as in our intestinal tracts]. Because they amplify themselves to high numbers, they can be thought of as a self-replicating antimicrobial.”
Additionally, the process by which the phages attach and lyse the bacteria of interest is quite rapid, notes Peterson.
Going from theory to practice
So, why don’t the phages just kill all of the bacteria? Unfortunately, there is great variation amongst bacteria, both in species and in subtypes, says Glisson. And phages tend to be specific for only a narrow range of bacteria types.
“For example, a particular phage may be able to lyse Salmonella typhimurium isolate A, but not Salmonella typhimurium isolate B, because the two isolates have some differences that give one the ability to resist the phage,” says Glisson.
If a processor wanted to kill all of the potential Salmonella types on a chicken carcass using phages, for example, he would have to have all of the phages specific for every potential type of Salmonella, says Glisson.
“This is technically very difficult, if at all possible, because of the huge variation amongst Salmonella and the huge variety of phages,” he says. “This is one of the most difficult technical problems that has limited the usefulness of phages so far.”
Similar to how bacteria develop resistance to antibiotics, concerns always remain about bacteria’s resistance to phages developing. To solve this issue, mixtures of phages are often combined into a single product.
“Each phage that comprises the mixture [there are usually six to eight phages in a mixture] needs to be individually approved, which entails determining the complete genetic sequence of the phage, demonstrating that the methods in which it is grown are safe, and demonstrating that human consumption of the phage will not lead to illness,” says Goodridge. “This explains the long [regulatory] approval process.”
In addition, they have a longer approval process because “the use of phages as antimicrobials is still in its infancy and because they are biological entities as opposed to chemicals,” says Goodridge. “The regulatory agencies are still a little unclear of how exactly to approve them for use.”
Peterson agrees that since bacteriophages are relatively new, that makes it quite challenging both for regulators and manufactures to work together to get through the approval process.
“There is no formal set of rules when seeking approval of a new bacteriophage, which can slow the process down,” she says.
Sorting out technical difficulties
Scientists and processors are still trying to understand the efficacy of bacteriophages, specifically how the use of bacteriophages fit into the food-safety system and impact pathogenic bacteria, notes Peterson.
“Secondly, you need a lot of bacteriophages to impact specific pathogenic bacteria, and the application of the intervention must be at a terminal point [just prior to slaughter] as to prevent other pathogenic bacteria from becoming immune to the bacteriophage itself,” she says.
And it remains to be seen where phages are most effective, whether they should be used with water and feed or on carcasses, says Gill. “They also need efficacy and market demand,” he says. And a lower price tag.
“It’s hard to say what the market value of phages will end up at; it depends on demand really,” says Gill. “I don’t think they’ve reached a peak economy of scale yet. It’s worth noting that phages could potentially be used in applications that other antimicrobials can’t be used in, due to concerns of efficacy, organoleptic qualities or residues.”
Indeed, so far phages carry a higher cost because the manufacturing and approval of this technology is quite challenging and time-consuming.
“Bacteriophages are expensive not only because of the manufacturing process, but also because many bacteriophages are needed to see statistical results,” notes Peterson.
Gill notes that at this point there are still many questions as to its best usage in pre- and post-harvest applications regarding when, how and how much should be used.
“It’s not clear yet how phages should be best used for practical purposes, not just in the lab,” he says. “There are phage products available now for use on packaged meats and cheeses, for example, but I couldn’t tell you how much industry adoption there has been, or what the industry experience has been.”
However, no one is counting out phages as a new component to food-safety plans.
“Phages will play an important role in the future as the technical difficulties involved with phage therapy are sorted out,” says Glisson.



Report Abusive Comment